
Furnace Brazing Stainless Steel (SST) in a hydrogen atmosphere and welding are common metal joining techniques we use every day at Altair Technologies. Unlike mild steel, SST is a low vapor pressure material and is frequently used in very high vacuum applications. The heating of SST causes Chromium Oxide to form on the surfaces of stainless components. This creates a poor wetting surface that prevents braze filler-alloys from flowing uniformly. We do not employe torch brazing or use brazing rods at Altair, we are 100% dedicated to furnace brazing SST in wet or dry hydrogen environments.
The decision to furnace braze in “wet” or “dry” Hydrogen can depend on a few important aspects such as the base metal or substrate materials being used and/or the filler alloy type, as well as the application or performance requirements. In cases where the removal of oxides is predominantly important or necessary, dry Hydrogen brazing is used and if the user is more concerned with the removal of hydrocarbon contaminants, it is advisable to braze in wet Hydrogen.

The chart below shows the temperature and/or dew point where the native oxides for various metals can be reduced [dew points below -60 °C are not achievable) [Source: Based on Bredzs, N., and C. C. Tennenhouse, 1970, Metal-metal oxide equilibria in pure hydrogen atmosphere, Welding Journal 49(5): 189-s-193-s].
- Cr2O3stable even in Dry H2
- Stainless Chart
- Metal-Metal Oxide Equilibria in H2/H2O Atmospheres
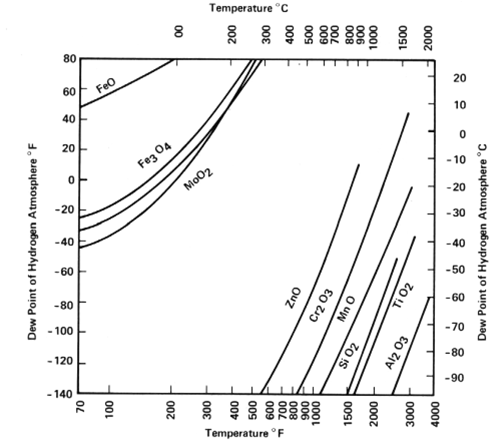
Chromium (Cr), which is a large constituent of stainless steel, occurs near the middle of the oxidation/reduction equilibrium space that hydrogen furnaces can produce. As desired, we can form Chromium Oxide or reduce that oxide by the atmosphere’s dew-point for temperatures greater than 800 ºC. When furnace brazing stainless steel that is rich in Chromium, we braze in a dry Hydrogen atmosphere which acts as a fluxing agent to reduce surface oxides and remove hydrocarbon contamination at brazing temperature, producing an ultra-clean raw metal surface. Stainless steel can also be plated with Nickel or another suitable metal and then be alternately brazed in a wet Hydrogen atmosphere.
For very high vacuum applications, assemblies containing a significant amount of stainless steel are sometimes run through a high-vacuum clean fire to help remove excess Hydrogen which certain vacuum systems find it difficult to pump. Hydrogen (H2) gas acts as a deoxidizing agent. Many oxides, like Fe, Cu, Ni, and Co are easily reduced by H2 whereas many others like Al, Be, Ti & Si can be very tenacious and will not braze or reduce properly in wet or dry H2. Here we have a reactive family of elements that may form undesirable compounds and are therefore typically brazed in high vacuum (Vacuum Brazing) or with other inert gases like Helium or Argon.



